how to find valence electrons on periodic table|Valence electrons (video) : iloilo Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In . The Laperal White House. Image adapted from: Joseph’s. The Laperal White House was originally home to the Laperal family in the 1930s amid the American Colonial period. However, it became a .
how to find valence electrons on periodic table,Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In .

Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p .how to find valence electrons on periodic table Explain the relationship between the chemical behavior of families in the periodic table and their valence electrons. Identify elements that will have the most similar properties to a given element. .Explanation of how to count valence electrons of an element using both the electron configuration and/or the position of the element on the periodic table.Valence electrons (video) Now that we've classified our elements into groups on the periodic table, let's see how to determine the number of valence electrons. And so for this video, we're only talking .
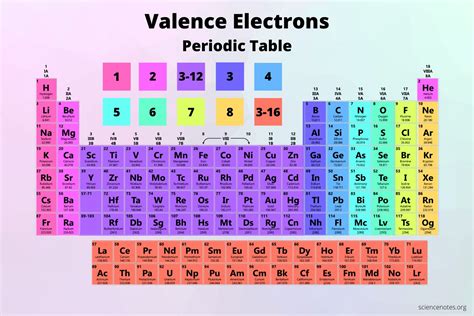
The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements .
The easiest way to find the number of valence electrons is to go by the element group in the valence periodic table. However, the most common method uses atom’s ground state electron .The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron . The most important electrons in an atom are the valence electrons, which are in the outermost energy level or shell. We'll look at how to determine the number of .Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table . For the main group elements (groups designated .

This chemistry video tutorial provides a basic introduction into valence electrons and the periodic table. It explains how to determine the number of valenc. Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .Characteristics of Valence Electron. Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Atoms are most stable if they have a filled valence shell .
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. (Where you will get the HD images along with the explanation). Valence Electrons Chart for All Elements. Atomic number Elements Valence electrons; 1: Hydrogen (H) 1: 2: Helium (He) 2: 3: Lithium (Li) 1: 4: Beryllium .how to find valence electrons on periodic table Valence electrons (video) The valence electrons are found based on where the element is on the periodic table. Locate the element on the periodic table. Counting along the row/period, count the number of boxes to your element. This will be how many valence electrons there are. For example: Carbon - there are four boxes in the row including the box with carbon . Example \(\PageIndex{1}\): Electron Configuration. Solution; The commonly used long form of the periodic table is designed to emphasize electron configurations.Since it is the outermost (valence) electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in the building .Elements: A pure substance composed of a single atom with a unique atomic number. Groups: The vertical column of the periodic table that signifies the number of valence electrons in an element. Periods: The horizontal rows in the periodic table that signify the number of electron shells in an element. Families: Elements that have the same . 1. By Using Periodic Table. It is the most widely used method to determine the number of valence electrons in an element. Here, we just refer to the periodic table and search for the position of the element in it. As we proceed downwards in a group, the numbers of valence electrons are same, although the number of shells increases. The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under . Such overlaps continue to occur frequently as we move up the chart. Figure 8.3.1 8.3. 1: Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale). . Valence Electrons and the Periodic Table. The number of valence electrons in an atom is reflected by its position in the periodic table of the elements (see the periodic table in the Figure below). Across each row, or period, of the periodic table, the number of valence electrons in groups 1–2 and 13–18 increases by one from one .
On the other hand, nitrogen can form NH 3 so it has a valence of 3, and 3 valence electrons. How many valence electrons does an element have? You can use the periodic table to help you determine how many valence electrons an element (specifically, a neutral atom of the element) has. Look at the group that the element is in, as the group . Elements in Group 17, known as halogens, have a valency of -1. They readily gain one electron to achieve a stable octet configuration. Group 16 elements, the chalcogens, have a valency of -2, and Group 15 elements, the pnictogens, have a valency of -3. These elements tend to gain electrons to achieve a stable electron configuration. We can find the number of valence electrons by observing the number of the group (vertical column) on the periodic table that contains the wanted element. For more clarification, it is known that: Group 1 — 1 valence electron. Group 2 — 2 valence electrons. Group 13 — 3 valence electrons.
Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second .
how to find valence electrons on periodic table|Valence electrons (video)
PH0 · What Are Valence Electrons? Definition and Periodic Table
PH1 · Valence electrons (video)
PH2 · Valence Electrons and the Periodic Table
PH3 · Valence Electrons
PH4 · How to Find Valence Electrons: 12 Steps (with Pictures)
PH5 · How to Find Valence Electrons: 12 Steps (with Pictures)
PH6 · How can I find valence electrons on the periodic table?
PH7 · Determine valence electrons using the periodic table
PH8 · Counting valence electrons for main group elements
PH9 · 11.1: Valence Electrons and the Periodic Table
PH10 · 10.6: Valence Electrons
PH11 · 1.3: Valence electrons and open valences